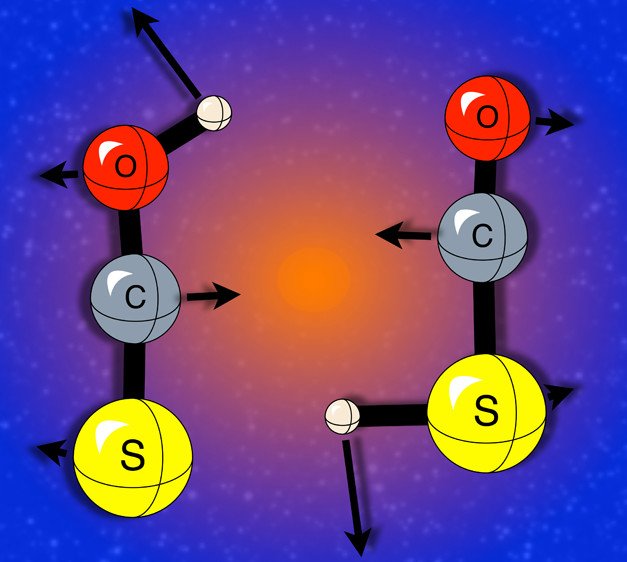
Credit: Fortenberry, R. et al. Journal of Physical Chemistry A 2012, 116, 38, 9582–9590
Quantum Chemistry
Quantum chemistry utilizes quantum mechanics to model how electrons behave within and between molecules. This is often done through computer codes that can handle the complicated mathematics involved. Quantum chemistry can determine what a molecule looks like, how it will probably react under specific conditions, and how it will absorb and emit energy when hit with light of various wavelengths. This provides astrochemists with much clearer pictures of the molecules on which they are working. It gives experimental chemists an idea of how reactions will progress and helps observational studies to determine what molecules might be present when the telescope is pointed at a certain object. These data can then provide the input parameters and molecular specifications used in astrophysical models and simulations.
The HOCS+ and HSCO+ cations, molecules with a positive charge, are important in interstellar chemistry especially in the formation of the detected interstellar molecule OCS. Through density functional theory (DFT) calculations, quantum chemist Ryan Fortenberry and colleagues provided the vibrational frequencies of these cations. Vibrational frequencies are often considered to be a molecule’s fingerprint. Such information is useful for other theoretical astrochemists to implement in their models and for observers to identify a detection of the molecule in space.
RESEARCH AREAS
RESEARCH METHODS